Abstract
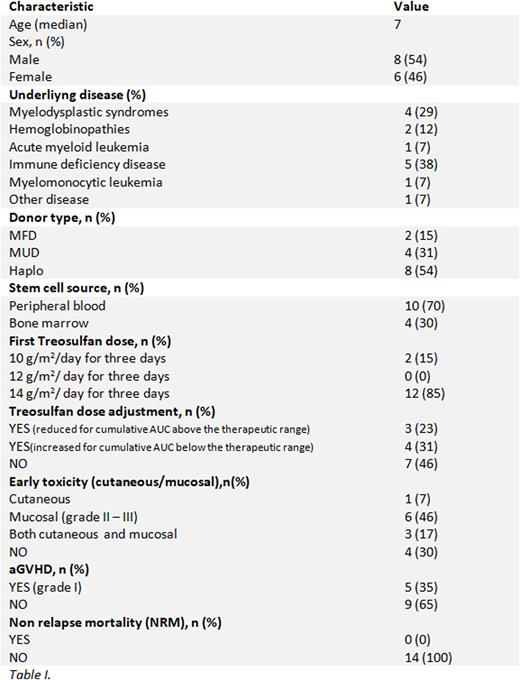
One of the major challenges to improving outcomes in hematopoietic stem cell transplantation is to choose the best conditioning regimen to reduce toxicity and Non Relapse Mortality, while maintaining adequate transplant efficacy. The use of Treosulfan, in combination with other chemotherapeutic agents, as part of the conditioning regimen, has progressively increased during the last decade, given its remarkable myeloablative and immunosoppressive properties and more favorable toxicity profile in comparison with Busulfan and Total Body Irradiation. For a therapy to be considered effective and safe, it is necessary to know its pharmacokinetic and pharmacodynamic properties, so that it reaches and maintains adequate concentrations in blood and tissues. Data on the pharmacokinetics of Treosulfan and the relationship between toxicity and efficacy in the pediatric population are still scarce, with reports of high inter-individual variability of Treosulfan concentrations in blood. The aim of this multicenter, prospective study is to develop a therapeutic drug monitoring system for Treosulfan dosing, in children undergoing hematopoietic stem cell transplantation for both malignant and non-malignant disorders, through the investigation of the pharmacokinetics in a large population of children after the first dose of Treosulfan, during the pre-transplant conditioning regimen (proposed cumulative therapeutic target, 'Area Under the Curve' (AUC) 4800 mg*h/L, range 3840 - 6000 mg*h/L). To achieve this aim, we measure the percentage of patients in therapeutic range, make dose adjustments if required, and define the relationship between Treosulfan exposure, early toxicity and efficacy, measured by time to engrafment and donor chimerism percentage after transplant. Of the 24 patients so far enrolled, the first 14 were analysed (Table I). Seven (54%) required a dose adjustment after the first administration of Treosulfan: reduced in three cases (43%) for a cumulative AUC above 6000 mg*h/L, increased in four (57%) for AUC below the therapeutic target, confirming the wide inter-individual variability. Enrollment continues, and follow-up of patients should allow the definition of the relationship between personalized Treosulfan dosing, toxicity and efficacy. Therefore, the development of a drug monitoring system for Treosulfan may be an important tool to optimize outcomes in the pediatric population.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.